2 The diagram below shows for a given temperature T a Boltzmann distribution of the kinetic energy of the molecules of a mixture of two gases that will react together such as nitrogen and hydrogen. The drawing below shows a mixture of molecules.
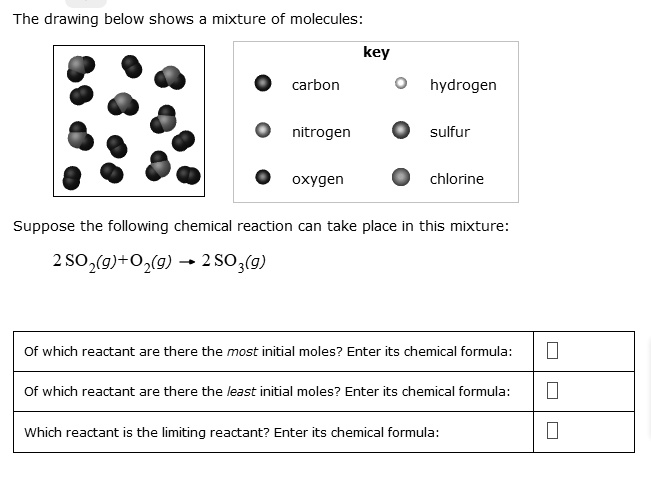
Solved The Drawing Below Shows Mixture Of Molecules Carbon Hydrogen Nitrogen Sulfur Oxygen Chlorine Suppose The Following Chemical Reaction Can Take Place In This Mixture 2so G 02 G 2 So3 G Of Which Reactant Are There
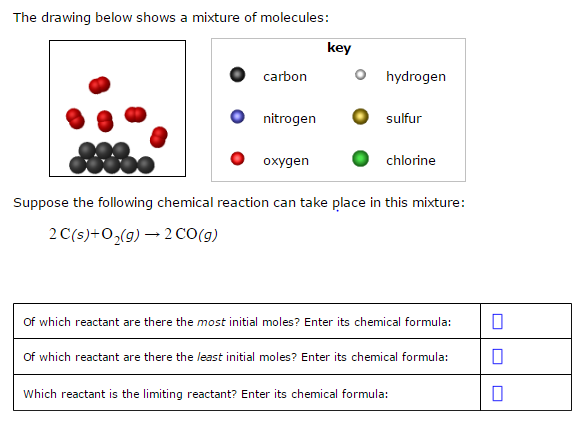
2C sO2 g 2 CO.
. The drawing below shows a mixture of molecules. Key carbon O hydrogen nitrogen O sulfur oxygen chlorine Suppose the following chemical reaction can take place in this mixture. The activation energy for the reaction Ea is marked.
Enter its chemical formula. 3 rows The drawing below shows a mixture of molecules. O CHEMICAL REACTIONS Identifying the limiting reactant in a drawing of a mixture The drawing below shows a mixture of molecules.
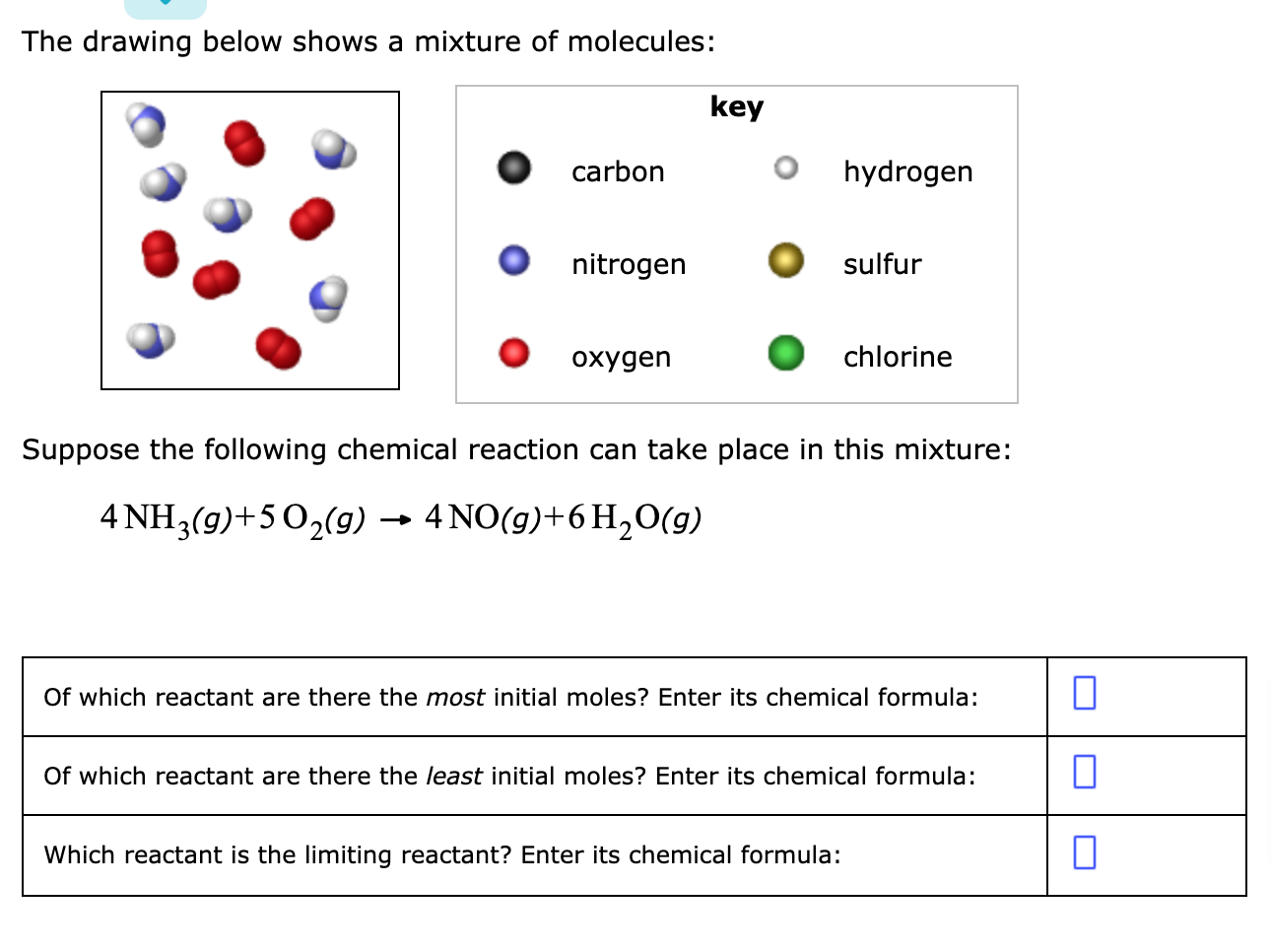
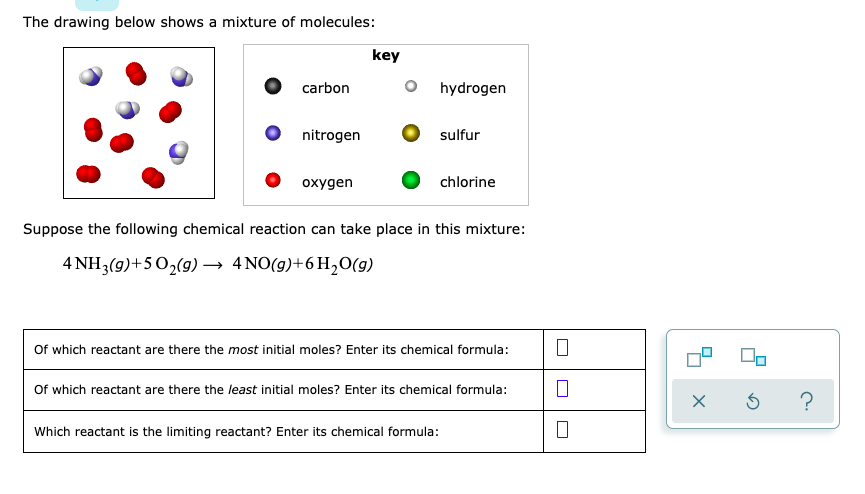
The dots represent molecules of a substance needed by the cell. 3H_2g N_2g rightarrow 2 NH_3g Of which reactant are there the most initial moles. The molecules are very small and hydrophobic.
Of which reactant are there the least initial moles. Start exploring Subjectschevron rightRESOURCESLiterature guidesConcept explainersWriting guidePopular textbooksPopular high school textbooksPopular ABusinesschevron rightEngineeringchevron rightLanguagechevron rightMathchevron rightSciencechevron. 12102016 ALEKS Student Name.
3 H gN2 9 - 2NH 9 Of which reactant are there the most ini. Key carbon O hydrogen nitrogen oxygen sulfur. The drawing below shows a simple way of purifying salt water what is.
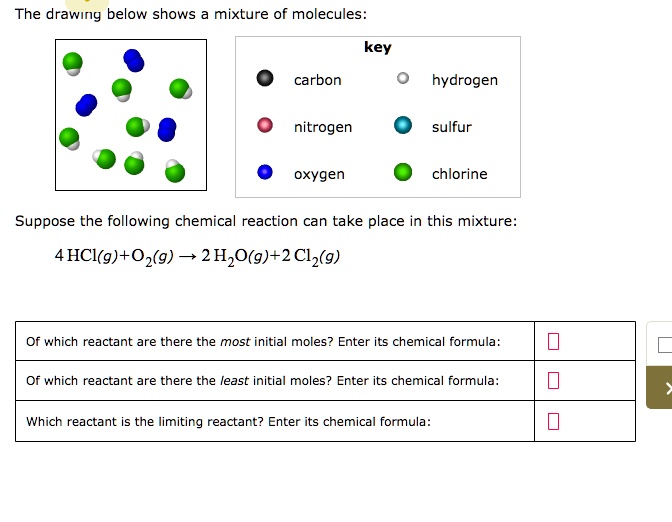
Iii Draw a second curve on the same axes and label it T 2 for the same mixture at a lower temperature. 4 HCl 9O2 g 2H2O g2012 9 of which reactant are there the most initial moles. Key carbon O hydrogen nitrogen oxygen sulfur chlorine Suppose the following chemical reaction can take place in this mixture.
Key carbon O hydrogen nitrogen sulfur oxygen chlorine Suppose the following chemical reaction can take place in this mixture. Shape of CCl 2 F 2 Shape of ClF 3 2 c Suggest the strongest type of intermolecular force between CCl 2 F 2 molecules. Include any lone pairs of electrons that influence the shape.
Suppose the following chemical reaction can take place in this mixture. Key carbon o hydrogen O nitrogen O sulfur oxygen chlorine Suppose the following chemical reaction can take place in this mixture. Hydrogen bond A is stronger.
Ii Explain the meaning of the term activation energy. The drawing below shows a mixture of molecules. COg2H29 - CH2OHg Of which reactant are there the most initial moles.
The drawing below shows a mixture of molecules. 4HClgO29- 2H2092 Cl29 Of which reactant are there the most initial. Suppose the following chemical reaction can take place in this mixture.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Which statement is correct. Up to 256 cash back The drawing below shows a mixture of molecules.
Key carbon hydrogen nitrogen sulfur oxygen chlorine Suppose the following chemical reaction can take place in this mixture. A large pool of water trapped in sand and gravel below the surface of the earth. The drawing below shows a mixture of molecules.
Up to 256 cash back The drawing below shows a mixture of molecules. 3 on a question The drawing below shows the fluid inside and outside a cell. MoreStudyResourcesWe got the study and writing resources you need for your assignments.
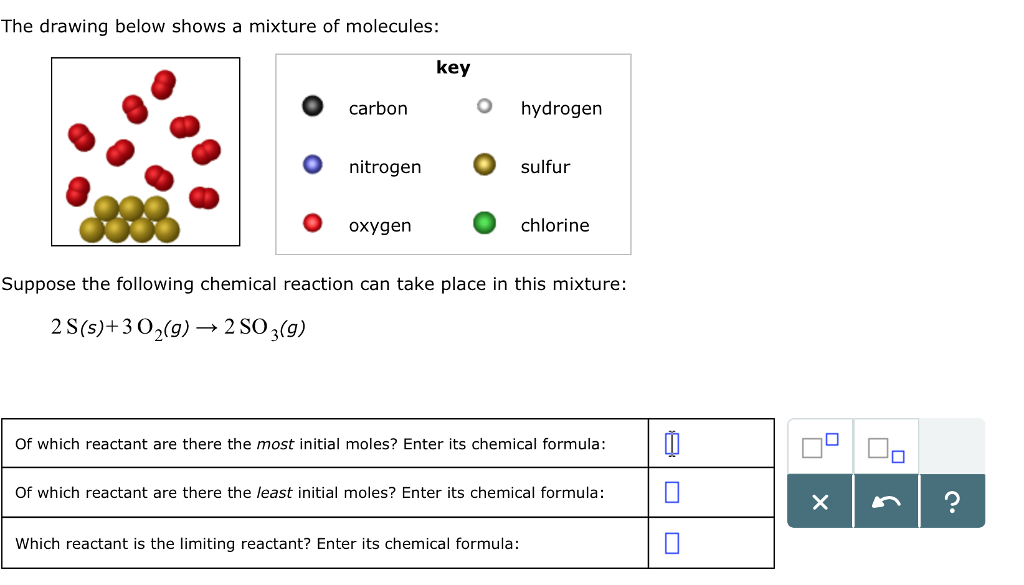
As per the equation 1 mole of Carbon will react with 2 moles of Oxygen. 2 Ss 3O2g rightarrow 2 SO_3g. Of which reactant are there the least initial moles.
Enter its chemical formula. CO 2H 9 CHOH 9 Of which reactant are there the most initial moles. Key carbon hydrogen nitrogen sulfur oxygen chlorine Suppose the following chemical reaction can take place in this mixture.
Enter its chemical formula. Key carbon hydrogen nitrogen sulfur oxygen chlorine Suppose the following chemical reaction can take place in this mixture. Iv By reference to the curves state and explain in molecular terms the effect of reducing the temperature on the rate of reaction.
II 0 0 of which reactant are there the least initial moles. Sulfur oxygen chlorine Suppose the following chemical reaction can take place in this. The drawing below shows a mixture of molecules.
A On the graph above. 2CsO2g 2 COg Of which reactant are there the most initial moles. The drawing below shows a mixture of molecules.
Gg Xg Yg a Draw on the axes below a Maxwell-Boltzmann distribution curve for a sample of G in which only a small proportion of molecules has energy greater than the activation energy Ea. Suppose the following chemical reaction can take place In this mixture. Enter its chemical formula.
02 Of which reactant are there the least initial. Gas G decomposes as shown in the equation below. 2 H_2 g O_2 g.
0 Of which reactant are there the least initial moles. Key carbon O hydrogen nitrogen O. The activation energy for the reaction Ea is marked.
CO 92 H 9 CH OH 9 Of which reactant are there the most initial moles. A i Label the vertical axis. The drawing shows two water molecules.
_____ 1 b Draw the shape of a dichlorodifluoromethane molecule CCl 2 F 2 and the shape of a chlorine trifluoride molecule ClF 3. The drawing below shows a mixture of molecules. G g g g Of which reactant are there the most initial moles.
Enter its chemical formua. Enter its chemical formula. The drawing below shows a mixture of molecules.
CS 2g3 O2g â CO2g2 SO2g of which reactant are there the most initial moles. Key O carbon O nitrogen O oxygen hydrogen O chlorine Suppose the following chemical reaction can take place in this mixture. Key carbon O hydrogen nitrogen O sulfur oxygen chlorine Suppose the following chemical reaction can take place in this mixture.
Ea energy number of molecules a On the graph above. In the diagram provided there are 8 molecules of C and 5. View the full answer.
Q2 Thediagram below shows for a given temperature T a Boltzmann distribution of the kinetic energy of the molecules of a mixture of two gases that will react together such as nitrogen and hydrogen. 12102016 Stoichiometry Identifying the limiting reactant in a drawing of a mixture The drawing below shows a mixture of molecules.
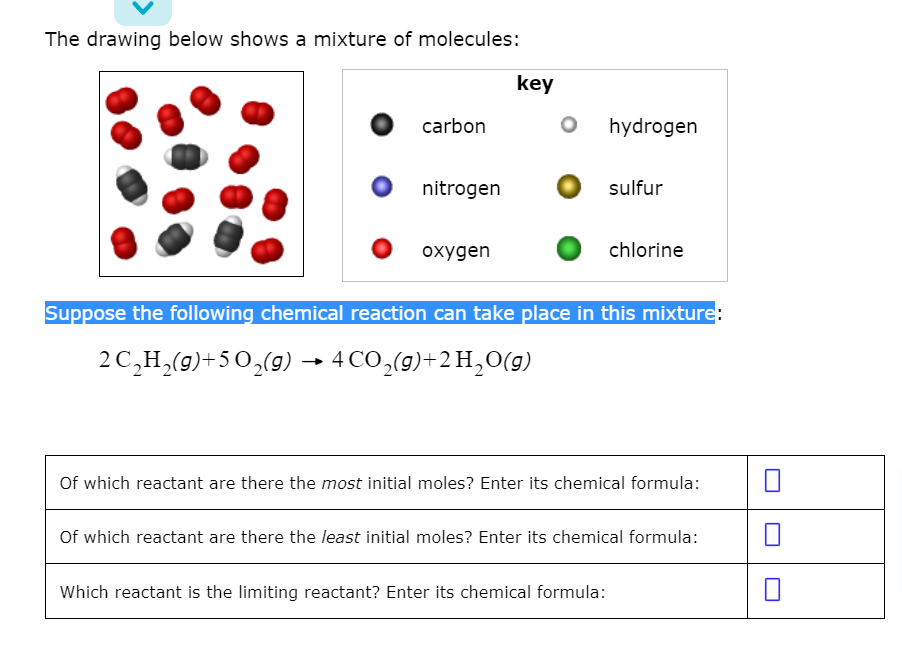
Answered The Drawing Below Shows A Mixture Of Bartleby

Solved The Drawing Below Shows A Mixture Of Molecules Key Chegg Com
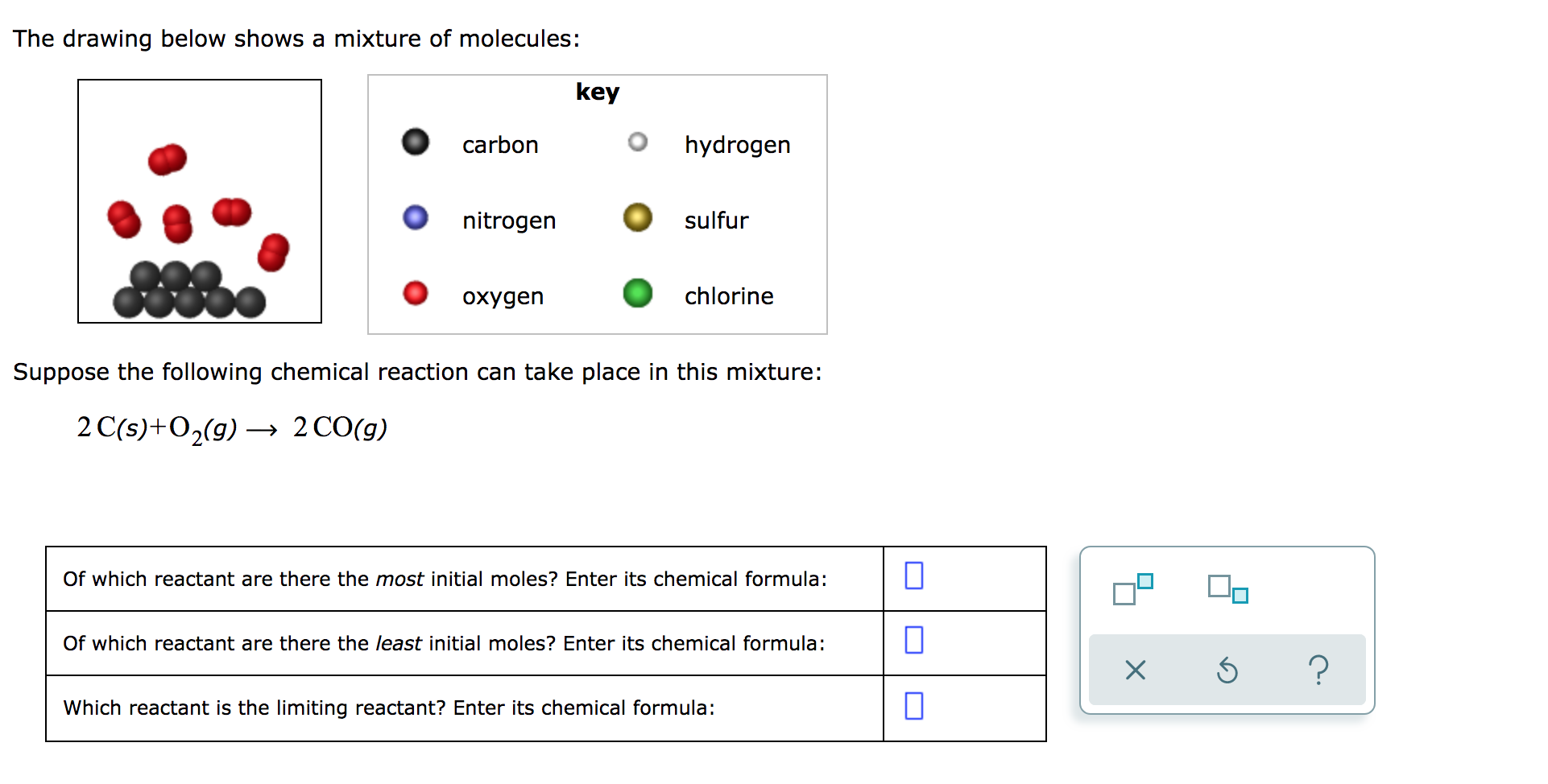
Solved The Drawing Below Shows A Mixture Of Molecules Key Chegg Com
Solved The Drawing Below Shows A Mixture Of Molecules Chegg Com
Solved The Drawing Below Shows Mixture Of Molecules Key Carbon Hydrogen Nitrogen Sulfur Oxygen Chlorine Suppose The Following Chemical Reaction Can Take Place In This Mixture Hc1 G 02 G 2 H20 G 2 Cl2 G Of Which Reactant

The Drawing Below Shows A Mixture Of Molecules Key Carbon Bud Hydrogen Nitrogen Sulfur Oxygen Chlorine Homeworklib
Solved The Drawing Below Shows A Mixture Of Molecules Key Chegg Com
Solved The Drawing Below Shows A Mixture Of Molecules Chegg Com
0 comments
Post a Comment